How to Handle Waste Generated from Surgical Procedures
In the UK, waste that derives from surgical procedures is broadly defined as clinical waste. Such waste must be disposed of in a specific waste container or receptacle during or immediately following the procedure.
Classification of waste is essential for proper storage, labelling, and disposal. It is the duty of care of any facility producing clinical waste to maintain and follow adequate disposal practices.
TOPICS WE WILL COVER:
1 / Identifying clinical waste from surgical procedures
2 / Duty of care with clinical waste
4 / Turn to Sharpsmart for guidance
Identifying clinical waste from the operating theatre
Identification of clinical waste sounds simple; however, clinical waste generated from surgical procedures comes in many forms, including anatomical, infectious, non-infectious, and hazardous waste, among others. Gauze, dressings, barrier wrap, gloves, and even surgical instruments are clinical wastes. 
Sharps waste, such as needles, scalpels, lancets, and glass are also generated within surgical procedures. Keep in mind that sharps can also be defined under other waste streams, such as infectious or cytotoxic/cytostatic, which require specific instructions for safer disposal. In cancer treatments, hazardous chemicals, such as cytotoxic and cytostatic medicines, are also types of clinical waste.
Anatomical waste can be defined as infectious or non-infectious as well. It is important to segregate or separate infectious from non-infectious materials, and the same applies to clinical waste.
Segregation is key to ensuring waste is sent to the proper disposal outlet. It is vital for clinical waste producers to know whether such waste is controlled due to its type or source. Turn to Schedule 1 of the Controlled Waste Regulations of 2012 (England and Wales) to determine whether your waste is controlled.
As a general rule, always keep in mind that clinical waste can be infectious, hazardous, or even radioactive, and always follow proper standards in the handling, storage, packaging, and labelling of such waste prior to disposal or transportation offsite.
Duty of care with waste from surgical procedures
The Department for Environment Food and Rural Affairs and the Environment Agency provide statutory guidance regarding duty of care for waste. The Code of Practice offers detailed guidelines on meeting waste duty of care requirements that can be found in the Environmental Protection Act 1990, section 34(7). In brief, duty of care in regard to clinical waste means that generators or producers of such waste ensure the safe management of that waste to not only protect human health but the environment.
Duty of care is not only assigned to waste producers, but also to households who are required to ‘take all reasonable measures’ that are available to ensure that waste is disposed of properly or transferred only to authorised persons.
In brief, duty of care for clinical waste requires that waste producers or holders meet a number of requirements in order to:
- Reduce the risk of unauthorised or harmful treatment or disposal
- Prevent breaches and require proper documentations for permits
- Ensure that hazardous waste is properly handled, stored, and labelled for transport and is always under their control prior to disposal
- Ensure that waste transfer processes are done correctly and with those who have gained authorisation
- Ensure accurate descriptions of the waste for transporters or ultimate disposal sites before waste leaves their property
It is the responsibility of the waste generator to ensure that all personnel that may come into contact with clinical waste know how to identify and segregate it from other waste streams. This not only ensures compliant waste processes but follows health and safety guidelines.
Containing clinical waste
Proper segregation and use of specific bags, containers, or bins for clinical waste can differ depending on the site producing that waste. For example, a dental practice, hospital, outpatient centre, or plastic or cosmetic surgery center, will all produce or generate varying types and volumes of clinical waste from surgical procedures. It is the responsibility of every such practice to correctly segregate, accurately label, and package waste appropriately for transportation. 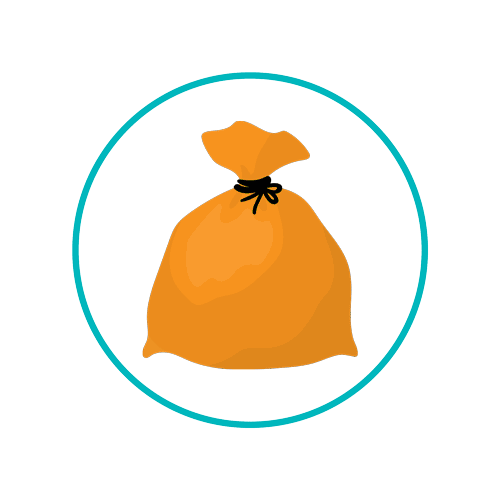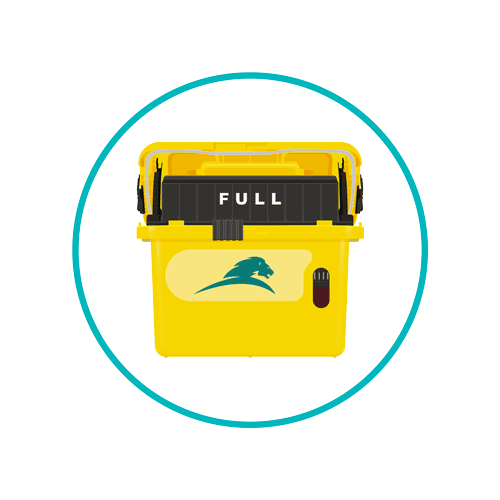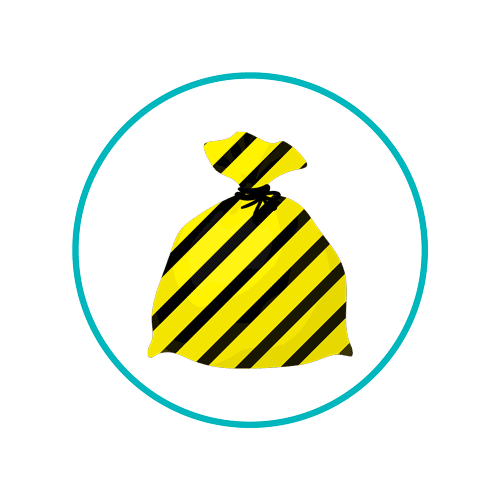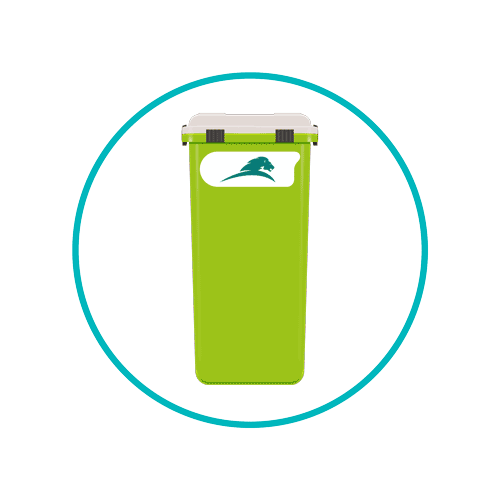
It is essential that every site, based on type of procedures conducted, be well versed in how such waste is to be contained and disposed of. For example, sharps are always to be placed in a sharps container. The type of sharps container (what colour lid) will depend on the nature of the patient (infectious or non-infectious) and what the sharp has been used for. For example, if the sharp is used to admininster cytotoxic or cytostatic medicines, it should be placed in a sharps container with a purple lid. Such receptacles must be leak-resistant, puncture-resistant, and resist crushing. Soft clinical waste such as contaminated PPE and other wastes that might present a risk of infection are to be placed in UN certified orange bags and labelled as hazardous. However, any such waste that has been contaminated by medicine or chemicals must be placed in a yellow UN certified bag.
Bandages covering an infected post-operative wound are to be disposed of in an orange bag, although the same bandages from a non-infectious wound can be placed in the offensive waste stream and disposed of in a yellow and black bag. Each also has different disposal routes.
Midwifery and hospital maternity centres must also properly contain anatomical waste. For instance, anatomical waste such as a placenta is to be placed in a red-lidded container and disposed by incineration.
In terms of disposal requirements, orange bags require specialist treatment to render infectious waste safe, whilst offensive waste can be used to generate electricity at waste to energy faciltiies, pushing it up the waste hierarchy.
A number of agencies and resources provide a wealth of information regarding clinical waste classifications, packaging, labelling, and disposal practices. For example:
- Health and Safety Executive – management of healthcare waste
- The Department of Health publication “Health Technical Memorandum 07-01: Safe Management of Healthcare Waste
- Waste Classification – guidance on the classification and assessment of waste
There is no excuse for not knowing the rules and regulations for compliant segregation and clinical waste management processes from generation to final disposition.
Turn to Sharpsmart for guidance 
Turn to Sharpsmart for guidance when it comes to segregation and waste disposal practices for clinical waste in order to maintain compliance and reduce the risk of fines or penalties. Such care also protects human health and the environment. Sharpsmart provides the latest in clinical waste technologies, comprehensive services, and educational resources regarding compliance and regulations to ensure that your facility complies with duty of care practices. For more information on our products or services, call us today.
Let's Talk!
Your time is valuable, and we don’t want to play hard to get. You can either phone us directly on the details listed on our contact page, or feel free to fill out this short form and one of our team members will get back to you as quickly as possible.
