2003, The Environmental Protection (Duty of Care) (England) (Amendment) Regulations, HMSO
1991, The Environmental Protection (Duty of Care) Regulations 1991, HMSO.
1990, The Environmental Protection Act, HMSO.
2011, Technical Guidance WM3: Hazardous Waste – Interpretation of the definition and classification of hazardous waste, Environment Agency, SEPA, NIEA
2009, Waste Batteries and Accumulators Regulations, HMSO
2009, The Hazardous Waste (England and Wales) (Amendment) Regulations, HMSO
2005, The Hazardous Waste (England & Wales) Regulations 2005, HMSO.
2005, Hazardous Waste – Interpretation of the definition and classification of hazardous waste, Environment Agency/Scottish Environment Protection Agency/Environment & Heritage Service.
2005, A Guide to the Hazardous Waste Regulations: Records, Registers and Returns, Environment Agency
2005, A Guide to the Hazardous Waste Regulations: Consignment Notes (version 1.8), Environment Agency
2002, The Control of Substances Hazardous to Health Regulations (COSHH), HMSO
1999, Control of Substances Hazardous to Health Regulations, Stationery Office.
1994, Health and Safety at Work etc. Act 1974, HMSO.
1992, Management of Health and Safety at Work Regulations 1992, HMSO.
EPR 5.07 – Clinical Waste Guidance
UN 3291, Clinical Waste (Bio Medical Waste or Regulated Medical Waste)
2011, The Waste (England and Wales) Regulations, HMSO
2011, Safe Management of Healthcare Waste England 2013 DOH.
2014, National Guidance for Healthcare Waste Water Discharges, Water UK – UK Water and Sewerage Services
2011, How to comply with your environmental permit. Additional guidance for: Clinical Waste (EPR 5.07), version 1.1, Environment Agency
2010, The Environmental Permitting Regulations (England and Wales), HMSO
2010, Guidelines on the Handling and Disposal of Hospital Pharmacy Wastes (For England and Wales and Information for Scotland and Northern Ireland), NHS Pharmaceutical Quality Assurance Committee
2008, The Health and Social Care Act: Code of practice on the prevention and control of infections and related guidance, Department of Health
2007, HTM 07-06: Disposal of Pharmaceutical Waste in Community Pharmacies, Department of Health.
HTM 07-01 2013: Safe Management of Healthcare Waste, Department of Health.
2005, Guidance for Dentists on Waste Dental Amalgam, DEFRA
2000, Healthcare Waste Minimisation – A Compendium of Good Practice, Department of Health
ISO 9001 (Quality Management Systems)
ISO 14001 (Environmental Management Systems)
OHSAS 18001 (Occupational Health and Safety Management)
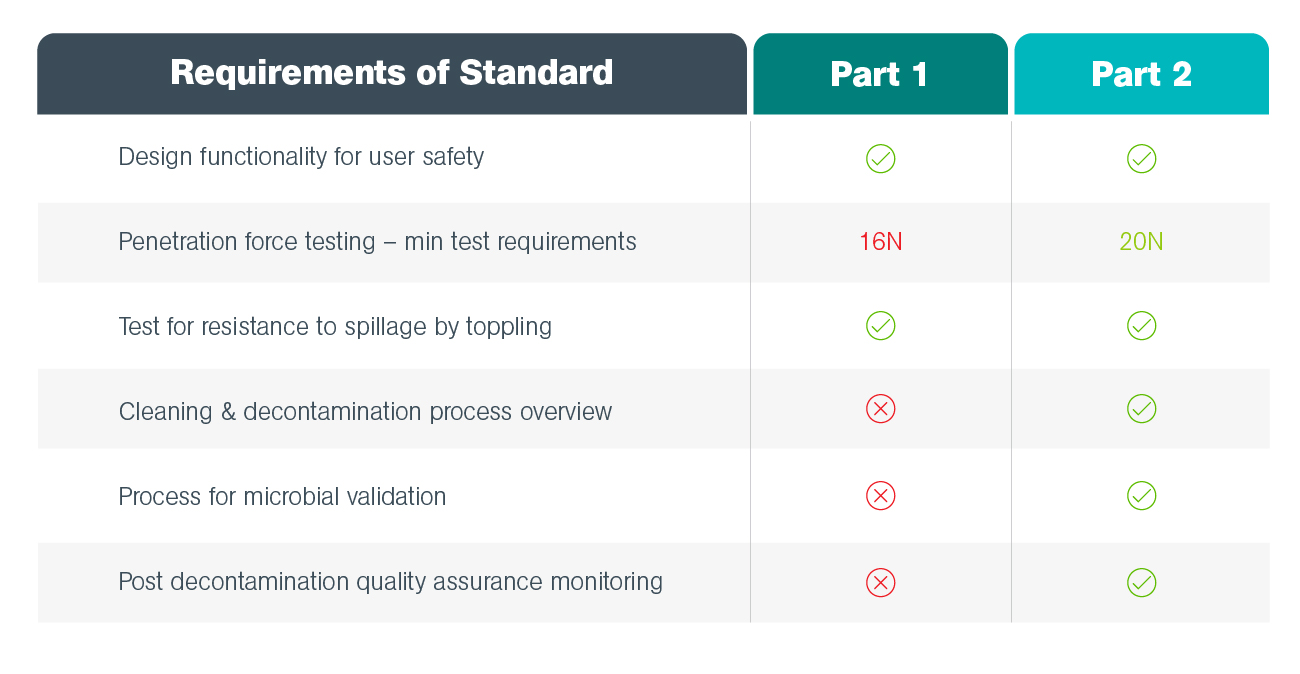
ISO 23907:2012 Sharps injury protection — Requirements and test methods — Sharps containers
2009, The Carriage of Dangerous Goods and Use of Transportable Pressure Equipment Regulations, HMSO (ADR)
2005, The Waste Management Licensing (England and Wales) (Amendment and Related Provisions) Regulations, HMSO
2005, The List of Wastes (England) Regulations, HMSO
2005, The List of Wastes (England) (Amendment) Regulations, HSMO
2005, The Landfill (England and Wales) (Amendment) Regulations, HMSO
2004, The Control of Substances Hazardous to Health (Amendment) Regulations, HMSO
1994, The Waste Management Licensing Regulations, HMSO
1992, The Controlled Waste Regulations, HMSO
2007, The Waste Electrical and Electronic Equipment (Amendment) Regulations, HMSO 2007, HTM 07-05: The Treatment, Recovery, Recycling and Safe Disposal of Waste Electrical and Electronic Equipment, Department of Health.
2006, The Waste Electrical and Electronic Equipment Regulations, HMSO