Sharps Removal: Best Practices

Does your facility practice compliant sharps removal methods? Best practices for sharps disposal and removal are required for the safety of healthcare providers, patients, and waste transporters. Sharps removal and disposal practices must not risk harm to others. It is the duty of care of every healthcare employee to be aware of the welfare and safety of others when it comes to sharps devices and their disposal.
Creating a safe environment for healthcare professionals is also essential in preventing sharps related injuries. Following the guidelines and engaging in best practices for sharps removal requires an integrated approach, knowledge of the rules, and training for all professional and ancillary employees in a healthcare environment.
TOPICS WE WILL COVER
3 / Documentation prior to sharps removal
4 / Sharps removal labelling requirements
5 / Managing compliance for sharps removal
Know the rules
Best practices and guidelines are designed to ensure safe sharps removal from initial use to ultimate disposal, reducing needlestick and sharps injuries throughout the UK.
Knowing the rules and regulations for sharps safety is the first step toward ensuring best practices are in place in any healthcare environment. From hospitals to private practices, care homes, cancer treatment centres, dialysis facilities, veterinary practices, and down to body piercing and tattoo parlours, safe sharps removal requires diligence.
The Health and Safety Executive Regulations of 2013 (Sharps Instruments in Healthcare) is a good starting point for awareness. The directive was created in order to reduce the risk of blood-borne infections and to prevent injuries among healthcare workers caused by sharps instruments, including needles. The document provides guidance in healthcare scenarios and defines legal obligations for both employees and employers.
The importance of compliant waste segregation and sharps safety can also be found in the publication of the Department of Health’s Health Technical Memorandum 07-01: Safe Management of Healthcare Waste.
Sharps waste bins 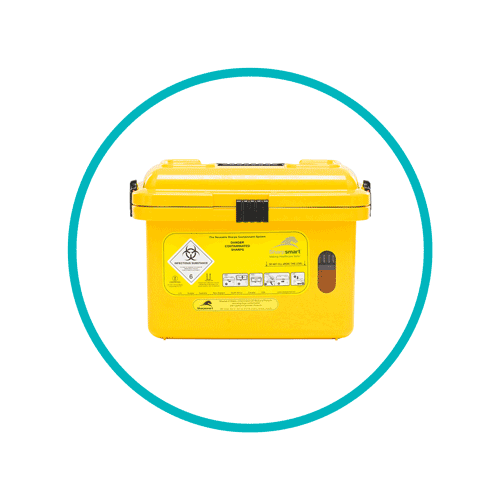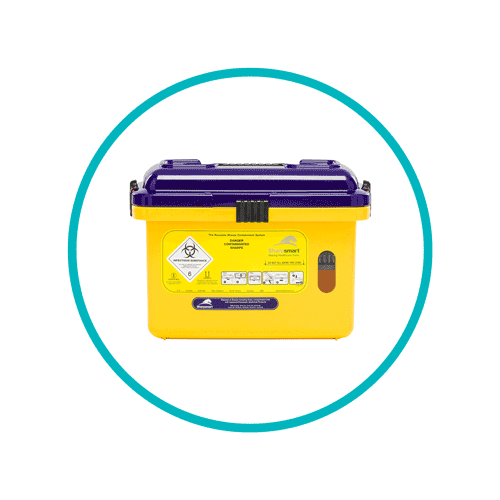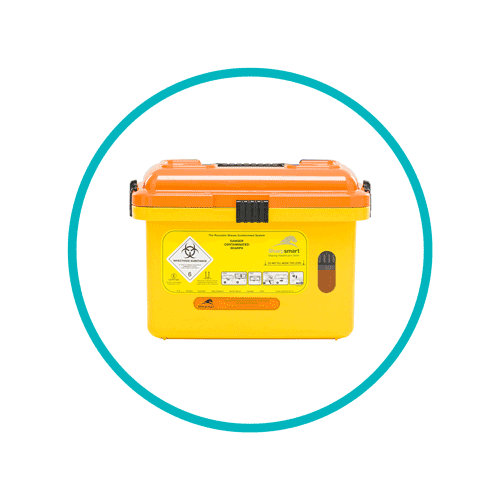
According to the Health Technical Memorandum, sharps waste is to be disposed of depending on its medicinal components. Yellow bins and receptacles are for sharps waste, and the colour of the receptacle lid or the receptacle itself is determined by potential contamination of various components. For example:
- Yellow lidded sharps containers are for sharps wastes contaminated with both medicinal and blood products. This waste stream should also contain fully discharged syringes (see section 5.46 of the memorandum). This waste stream should not be used for the disposal of cytotoxic and cytostatic medicines. For Sharpsmart customers, waste in yellow lidded sharps containers are disposed of by our patented no-burn technology.
- Purple lids denote sharps waste that has been contaminated with cytotoxic or cytostatic medicinals. Sharps removal and disposal for this type of waste is destined for incineration by authorised facilities.
- Orange lids are designed for sharps that have been contaminated with blood, but not medicines. Such sharps waste can be removed and destined for either incineration or disinfection through alternative treatments as long as such treatments are done at an authorised facility.
Sharps bins must comply with NHS standards and be made available at the sharp’s point-of-use whenever possible. Approved sharps containers must be resistant to leakage or penetration and have a lid and closure options that prevent spillage. In most cases, sharps containers or bins should be wall-mounted for safety. Do not fill above the fill line. Once the container is full, it should be locked or firmly sealed, signed, dated, and then affixed with an identification tag designating its contents. It should then be placed or stored in a secure area to await collection and transport.
Once collected, most sharps waste is disposed of through incineration; however, Sharpsmart has pioneered a non-burn process that safely decontaminates sharps waste for disposal without the negative environmental impact assoicated with operating incinerators.

Documentation prior to sharps removal
Removal of healthcare or medical waste from any facility, especially sharps, should be documented. This is essential for compliance. Prior to transportation or off-site sharps removal practices, documentation, packaging, and labelling must be compliant with legal guidelines.
Adhere to the guidelines of the Carriage of Dangerous Goods (CDG) transportation regulations. Documentation is more likely to be required to comply with hazardous waste regulations. For sharps removal and disposal, ensure that the sharps bin has the date as well as signature of the person or persons responsible for ensuring that compliance has been met prior to transportation off-site.
A number of procedural documents may be required to monitor compliance depending on the scenario. Individuals that may be involved in such scenarios include those responsible for specific areas or wards within hospital environments such as senior nursing or department managers, as well as infection control teams.
Sharps removal labelling requirements
All medical facilities, including hospitals, clinics, surgery centres, pharmacies, and veterinary services, are responsible for ensuring compliant transportation of sharps off-site. This duty of care is on the facility. It is also their responsibility to ensure that consignors are also compliant with transportation and disposal guidelines.
Sharps removal requires compliant classification and identification of contents, which also means clear marking and accurate labelling. Prior to sharps removal, containers should be clearly identified with the universal biological hazard sign as well as marked ‘Danger, contaminated sharps only, to be incinerated’.
Before sharps removal, it is important to ensure that the bin or receptacle contains only solids and does not contain more than a few milliliters of liquid residues. Never pour liquids from partially used vials or discharge components of a syringe into sharps receptacles as this is not in compliance with regulations.
Managing compliance for sharps removal
Managing compliance when it comes to sharps removal and disposal processes requires education, training, and knowledge regarding duty of care and familiarity with documentation requirements, packaging, and disposal methods.
Knowledge of such guidelines not only reduces the risk of sharps injuries in healthcare environments, but reduces the risk of contamination to anyone who comes into contact with such waste. It is vital that all healthcare facility employees, from top management to estates and facilities teams, know the rules regarding sharps removal to ensure safety.
That’s where a dedicated healthcare waste management provider like Sharpsmart can play a key role.
When it comes to sharps removal and disposal processes, safety comes first. Following best practices for sharps removal ensures safety and reduces the risk of injuries as well as fines and penalties for non-compliance. For more information about products, bins, or containers as well as compliant processes and procedures to ensure that your facility is compliant, contact a Sharpsmart representative today.
Let's Talk!
Your time is valuable, and we don’t want to play hard to get. You can either phone us directly on the details listed on our contact page, or feel free to fill out this short form and one of our team members will get back to you as quickly as possible.
